Introduction: Graft-versus-host disease (GvHD) is a multi-systemic disorder affecting patients who undergo allogenic hematopoietic stem cell transplantation (allo-HSCT) and is associated with considerable morbidity and mortality both in its acute (a) and chronic (c) forms, particularly in patients with steroid-refractory (SR)-GvHD (Flinn AM and Gennery AR. Fac Rev 2023).Given the lack of real-life data on GvHD, this study was conducted using a large European database with the objective to provide evidence on the epidemiology and treatment patterns in patients with aGvHD, cGvHD, and SR-GvHD.
Methods: This retrospective, observational cohort study was conducted between July 1, 2014 and December 31, 2020 in allo-HSCT patients registered to the European Society for Blood and Marrow Transplantation (EBMT) database. Male or female patients aged ≥12 years at index
(≥18 years for SR-GvHD and ruxolitinib [RUX] cohorts) who had received ≥1 allo-HSCT during this study period (January 1, 2017 to July 1, 2019 for SR-GvHD and RUX cohorts) were included in the study population. The EBMT cohort included all allo-HSCT patients, irrespective of GvHD development post-transplant (Tx). The primary objective was to assess the cumulative incidence of aGvHD and cGvHD in patients from the EBMT cohort who met the inclusion criteria. Overall survival of patients up to 60 months was also assessed in the EBMT cohort. The SR-GvHD cohort comprised a sub-selection of GvHD patients (from selected EBMT centers) who were diagnosed with SR-aGvHD, SR-cGvHD, or both, some receiving RUX as treatment; from the SR-GvHD cohort, a sub-selection of patients who had been treated with RUX for aGvHD and/or cGvHD, irrespective of treatment line, were included in the RUX cohort.
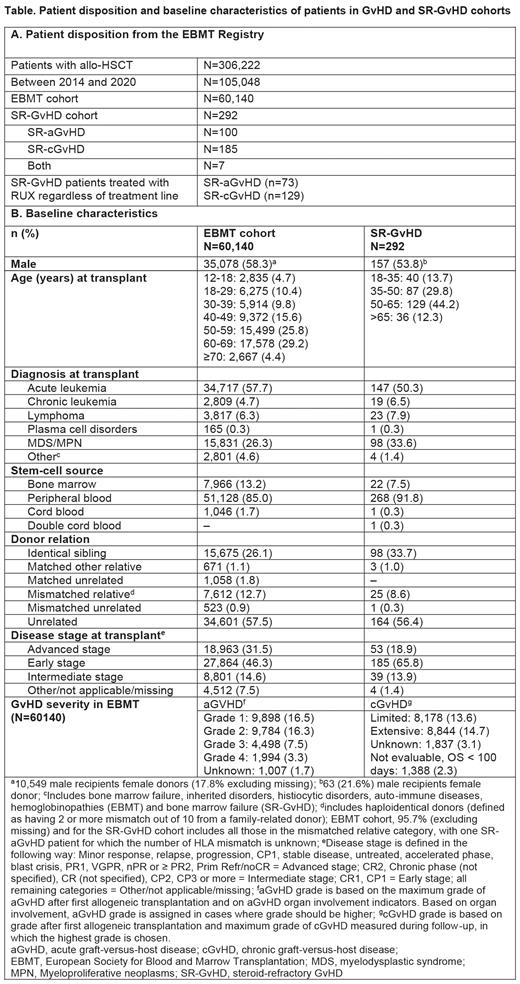
Results:In the EBMT database, 60,140 patients had received an allo-HSCT. Most patients (94.5%) underwent 1 allo-HSCT, the remaining had >1 procedure. The EBMT cohort included a higher proportion of male patients between 50 and 69 years (Table 1). The main indication for allo-HSCT was acute leukemia. At the time of Tx, most patients were in their early disease stage and had unrelated donors(Table 1). The cumulative incidence of aGvHD was 27.9% and 27.6%, 4 months after first and second Tx, respectively. The cumulative incidence of cGvHD was 37.4% and 29.7%, 60 months after first and second Tx, respectively. aGvHD was of grade 2-4 in 27.1% of patients, and the most affected organ was skin (33.6%, grade 2-4: 19.5%) followed by liver (4.0%, grade 2-4: 2.3%) and gut (4.6%, grade 2-4: 2.8%); however, organ involvement was not collected in many patients. The cGvHD was limited (13.6%), extensive (14.7%) or of unknown grade (3.1%). Graft failure occurred in 5.9% of patients. The overall survival of EBMT cohort patients at 60 months was 53.1% with a median follow-up time of 32.9 months. The most frequent cause of death was relapse/progression (30.6%) followed by infection (27.1%) and GvHD (19.1%).
The EBMT registry contains more SR-GvHD patients but only for subset of 292 SR-GvHD patients, additional information was obtained, of which 37 (34.6%) patients were treated with RUX and 70 (65.4%) with other second-line (2L) treatment for SR-aGvHD. For SR-cGvHD, 59 (31.9%) received RUX and 126 (68.1%) other 2L treatment. Baseline characteristics were comparable between SR-GvHD and the EBMT cohort (Table 1). Within SR-GvHD treatment groups, overall, the baseline characteristics were comparable across RUX and other 2L, and consistent with the EBMT and SR-GvHD cohorts. Compared with other 2L treatment, a slightly higher proportion of RUX-treated patients were females in SR-aGvHD cohort and males in SR-cGvHD cohort. While the most common first-line treatment for aGvHD in both RUX and the other group was methylprednisolone, for SR-cGvHD, it was prednisone+cyclosporine in RUX and prednisone in the other group.
Conclusion:This large retrospective real-world evidence study from the EBMT reports on patients with GvHD after allo-HSCT. The cumulative incidence of aGvHD at 4 months post-Tx and cGvHD at 60 months was similar after the first or the second Tx. Though this study provides valuable insights on the epidemiology and treatment patterns of SR-GvHD patients, interpretation of these data is limited due to the small sample size of RUX and other treatment groups compared with the EBMT cohort.
Disclosures
Avenoso:Pierre Fabre, Sanofi and Pfizer, Jazz Pharmaceuticals, Mallinckrodt/Therakos: Honoraria; Mallinckrodt/Therakos, Sanofi: Research Funding. Brossart:BMS, AstraZeneca, MSD: Honoraria, Other: Advisory Board; BMS: Research Funding. Gavriilaki:Alexion, AstraZeneca, Omeros, Sanofi, Sobi: Honoraria; Pfizer and Jazz Pharmaceuticals: Research Funding. Mico':Novartis: Honoraria. Martínez:Novartis: Honoraria, Research Funding. Ladetto:Novartis: Honoraria. Beguin:Galapagos: Research Funding. Klein:Amgen: Honoraria. Gunes:Novartis: Current Employment. Bobirca:Novartis: Current Employment. Penack:Equillium Bio, Jazz, Gilead, Novartis, MSD, Omeros, Priothera, Sanofi, Shionogi and SOBI: Membership on an entity's Board of Directors or advisory committees; Incyte and Priothera: Research Funding; Gilead, Jazz, MSD, Novartis, Pfizer and Therakos: Honoraria, Other: Travel support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal